Bonding
Atoms can exist alone, but more often they combine into compounds that can do many things. Some compounds are enormous, like DNA. Others are needed for everyday life, like water.
Bonding is all about the interactions of electrons. Some atoms give electrons away. Others take them. Some share them. Different situations may call for the electrons to do different things. It's all a matter of chemistry.
There's a lot more to it than simple rules, but these basics are the foundation.
There are three types of bonds to know, and they are based on the metallic properties of the atoms. Remember, metals are to the left of the stairs (on the periodic table). Non-metals are to the right of the stairs. Semi-metals (or metalloids) touch the flat parts of the stairs.
| Type of Bond | Types of Elements | Description of Electrons | Other Information |
| Metallic | Metal and Metal | Sea of mobile electrons | Allows for electricity and magnetism |
| Ionic | Metal and Non-Metal | Transfer of Electrons | Metals give electrons (become "+" cations) Nonmetals take electrons (become "-" anions) Semi-metals (metalloids) will generally form IONIC bonds. |
| Covalent | Non-Metal and Non-Metal | Sharing of Electrons | Can be single, double, and triple bonds |
| None | Anything with a Noble Gas | Noble Gases already have a full outer shell | Noble Gases Do Not React (under normal conditions) |
Practice Problems
| sodium and chlorine | IONIC (metal and non-metal) | lithium and calcium | METALLIC (metal and metal) |
| carbon and oxygen | COVALENT (non-metal and non-metal) | magnesium and argon | NO BOND (metal and noble gas) |
| iron and oxygen | IONIC (metal and non-metal) | molybdenum and chlorine | IONIC (metal and non-metal) |
| lithium and fluorine | IONIC (metal and non-metal) | nitrogen and carbon | COVALENT (non-metal and non-metal) |
| copper and zinc | METALLIC (metal and metal) | bromine and bromine | COVALENT (non-metal and non-metal) |
| neon and helium | NO BOND (noble gas and noble gas) | uranium and oxygen | IONIC (metal and non-metal) |
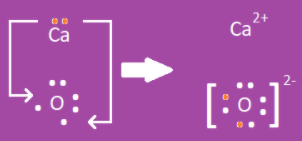
Drawing Ionic Bonds
- Start with Lewis dot diagrams of the elements that are bonding.
- Move electrons from the metal to the nonmetal.
- Write the positive charge to the top right of the metal.
- This is equal to the number of electrons lost.
- Place the nonmetal in square brackets.
- Outside the brackets in the top right corner, write the negative charge.
- This is equal to the number of electrons it gained.
Example 1: Potassium (K) and chlorine (Cl)

Example 2: Calcium (Ca) and oxygen (O)
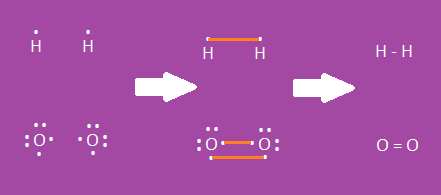
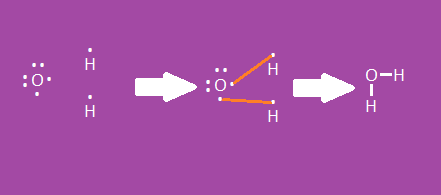
Drawing Molecular Bonds
- Start with Lewis dot diagrams of the elements that are bonding
- For molecular (covalent) bonds, show shared e- with dashes
- H and H become H-H
- O and O become O=O
- H and O become H-O-H
 |
 |
Bond Polarity
- Polarity occurs when a molecule has slightly charged ends, or is in an unbalanced structure
- Electronegativity: the willingness or ability of an element to draw electrons toward it
- Fluorine has the greatest EN (4.0)
- Francium has the least EN (0.7)
- Reference Table S shows electronegativities
- In a bond, a partial negative charge forms on the element with higher EN
- A partial positive charge forms on the element with a lower EN
- Larger differences in ENs means higher degree of polarity
- When two nonmetals with different electronegativities (EN) bond, they form a polar covalent bond
- Ex: C-H
- carbon has an EN of 2.6
- H has an EN of 2.2
- the carbon side is slightly negative
- Ex: C-H
- When two nonmetals with the same electronegativity bond (i.e. two of the same element = diatomic elements)
they form a nonpolar
covalent bond
- Ex: H-H
- electronegativities are the same
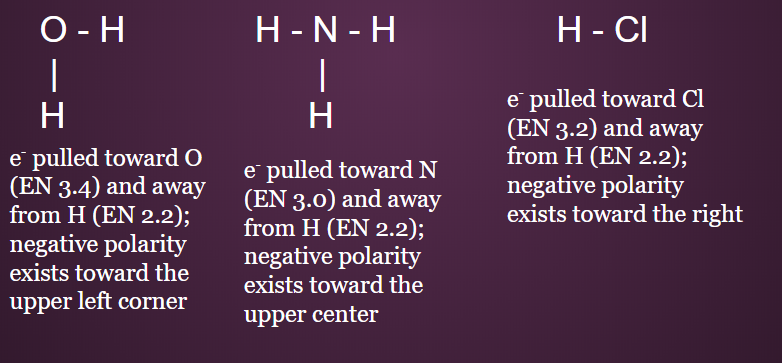
Asymmetrical Molecules
- ASYMMETRICAL molecules are POLAR because the pulls and pushes DO NOT cancel out
- Ex: H2O, NH3, HCl
Symmetrical Molecules
- Completely SYMMETRICAL molecules are NONPOLAR because the pulls and pushes DO cancel out
- Ex: CO2, CH4, diatomic elements (H2, O2, etc.)

Intermolecular Forces
- When a molecule is polar, it leads to intermolecular forces
- these are attractions between molecules
- In water, for instance, there is a negative pull toward oxygen and a positive shift on hydrogen
- this means hydrogens from one molecule will be drawn toward oxygens of another molecule
- this is called hydrogen bonding and is rather strong
Oxidation Numbers
- an oxidation number is the charge that is formed when an element forms an ionic bond
- the charge depends on how many valence electrons the element will either give or take from another element
- if an element gives away an electron, it becomes positively charged "+" (cation)
- if an element takes an electron, it becomes negatively charged "-" (anion)
- YOu can use your Reference Table's Periodic Table to find oxidation numbers.
- Some elements have multiple oxidation states. When in doubt, use the top number.
Example: Calcium has 2 valance electrons. It is easier for calcium to lose 2 electrons than it would be to gain six electrons. Therefore, calcium would get a 2+ charge.
| Group # | 1 | 2 | 3-12 | 13 | 14 | 15 | 16 | 17 | 18 |
| # Valence electrons | 1 | 2 | ----- | 3 | 4 | 5 | 6 | 7 | 8 |
| Oxidation # | 1+ | 2+ | ----- | 3+ | 4+ / 4- | 3- | 2- | 1- | 0 |
Crossing Over
- To find out what compound will be made when two elements react, you can either draw the dot diagrams and try
to pair up unpaired electrons.
- This can become messy and confusing.
- There is an easier way. All you need is the oxidation number for the elements you are combining.
- Then you cross over the numbers to get the subscripts. Subscripts just tell you how many of that element is in a compound.
- Subscripts are not written with + and - signs.
NOTE: This process is best for ionic compounds, but it will also work for covalent compounds.
Example: hydrogen and oxygen
| Hydrogen (group 1) and Oxygen (group 16) | Cross over the numbers (not the +/- signs) | This is what you get | Don't write the number "1" if you have it |
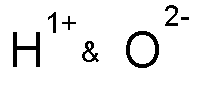 |
 |
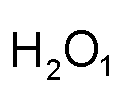 |
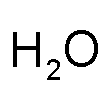 |

Practice problems
What compound forms when the following elements react?
| magnesium and chlorine | MgCl2 | potassium and nitrogen | K3N |
| rubidium and sulfur | Rb2S | calcium and bromine | CaBr2 |
| boron and hydrogen | BH3 | strontium and phosphorous | Sr3P2 |
| magnesium and oxygen | Mg2O2 (really it's MgO) | beryllium and argon | No reaction; argon is a noble gas |
Reverse Crossing Over
- Start with a binary compound
- Move the subscripts across and up to the opposite element
- Metals become + and nonmetals become -
- You can use this to determine which group the element is from
- Ex: oxidation 2+ is from group 2
- Ex: oxidation 1- is from group 17

Naming Binary Compounds
- To name binary compounds (IUPAC system):
- Name the metal
- Name the nonmetal, but change its ending to “ide”
- For polyatomic ions, don't change the ending
- Examples:
- NaCl = sodium and chlorine → sodium chloride
- Ca3P2 = calcium and phosphorous → calcium phosphide
- Al2(SO4)3 = aluminum and sulfate → aluminum sulfate (*polyatomic ion)
Polyatomic Ions
- Polyatomic ions (P.A.I.)
- Ions that are made up of several different elements (“poly” = many, “atomic” = atoms)
- Often treated as a single “element”
- Reference Table E
| NH4+ ammonium | NO3- nitrate | ClO3+ chlorate | SO42- sulfate |
| OH- hydroxide | CO32- carbonate | C3H2O3- acetate | PO43- phosphate |
Hydrates
- Hydrates are molecules that contain water: H2O
- Example: CuSO4 • 5H2O
- copper sulfate pentahydrate
- C6H12O6 (sugar) is a carbohydrate
- There are 6 carbon atoms
- There are 6 “water” molecules

Formula Types
- Molecular formula
- Precise ratio of elements in a compound
- C4H8
- Precise ratio of elements in a compound
- Empirical formula
- Simplest ratio of elements in a compound
- C4H8 → CH2
- Simplest ratio of elements in a compound
- Structural formula
- Shows how bonds are arranged
