Electron Configuration
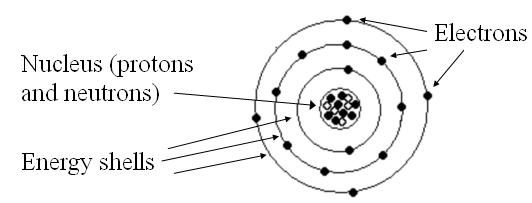
The Bohr model of the atom states that:
- The center of the atom is nucleus
- Protons and neutrons are found inside the nucleus
- Electrons are outside the nucleus, arranged in specific energy shells
- Note: Protons, neutrons, and electrons are composed of even smaller particles known as quarks. But those are beyond the scope of this class.
| Part of Atom | Definition | Analogy |
| energy shell | ring of electrons, related to period number | a bookcase |
| sublevel | part of an energy shell | shelves on the bookcase |
| orbital | part of a sublevel, holds 2 electrons | like sections on a shelf |
| electrons | subatomic particle "held" in the orbital | like the books in each compartment |
Basic Electron Configuration
Ground State
Electrons in the ground state are at the lowest possible energy level with no "gaps." This is how most atoms exist.
Your periodic table shows the electron configuration for electrons in the ground state.
Example: sodium: 2-8-1
This says there are 2 electrons in the first ring, 8 electrons in the second ring, and 1 electron in the third ring. Sodium has 11 total electrons.
Notice how this configuration also tells you how many rings of electrons the atom has.
Excited State
Sometimes an electron receives a packet of energy that pushes it to the excited state. This means (at least) one electron is further away from the nucleus than normal.
Example: sodium: 2-7-2
Normally, sodium has 8 electrons in the second ring, but this shows only 7, while the outer ring now has 2 electrons (instead of the normal 1). This means one electron "moved up" to another ring and now in the excited state.
Notice how the total number of electrons (11) is the same as sodium in the ground state.
Switching Between States
Electrons can only move between states when an exact amount of energy is absorbed. This amount differs for every atom.
When an electron is in the excited state, it drops down to the ground state and releases a photon, which is a packet of light energy. This produces a specific color.
Every element produces its own pattern of colors (like a fingerprint), which allow us to identify an element based on its bright line spectrum.
A bright line spectrum is a set of colors given off by a given element.
Detailed Electron Configuration: Orbital Notation
Sublevel Chart
| Sublevel name | Number of orbitals | Maximum number of electrons |
| s | 1 | 2 |
| p | 3 | 6 |
| d | 5 | 10 |
| f | 7 | 14 |
Electrons fill up from the lowest energy to the highest energy, but this is not always in number order. You must put the sublevels into order from low to high energy first. This is done by creating the sublevel energy chart, as shown below.
To create it:- Write the numbers 1 through 7 in a vertical line
- Next to each number, write the sublevels contained within.
- Always start with "s"
- The number of the energy level equals the number of sublevels it can hold
- So, energy level 1 only has 1 sublevel (s)
- Energy level 2 has 2 sublevels (s and p)
- Energy level 3 has 3 sublevels (s, p, and d)
- And so on...
- For our purposes, we will only use s, d, p, and f, even for levels that have g, h, i.
- Diagonally draw arrows down and to the left so that you hit each sublevel.
- Make a list of each sublevel as you hit it
- Keep your lines straight
- Don't miss any
- You can stop at 7s. We won't go higher than that.
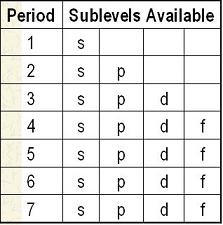
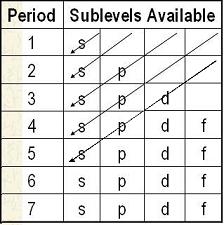
Creating the Sublevel List
 |
 |
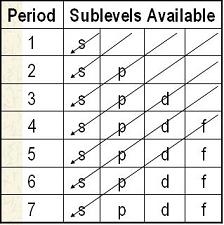 |
| Start with the full chart | Draw diagonal lines from top right to bottom left. Make a list of the order of sublevels as you go. 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s... |
At the end, you will have all the sublevels in order from lowest to highest
energy. 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s |
Creating the Electron Configuration
- To create an electron configuration, you need to first know the number of electrons.
- Always start from the lowest energy sublevel (1s) and fill them up until you run out of electrons.
- Remember, s has 1 orbital, p has 3, d has 5, and f has 7.
- Each orbital can fit 2 electrons.
- Always maximize the spin when filling a sublevel.
- So, one electron goes into each of the three p orbitals before they double up.
- These are called Ground State electron configurations because they are created by using the lowest energy sublevels first.
EXAMPLE
18OLook up oxygen to find the number of protons (atomic number). That will equal the number of electrons in a neutral atom. Oxygen has an atomic number of 8, so there are 8 protons and also 8 electrons. There are 10 neutrons in this example because 18-8 = 10.
↑↓
↑↓
↑↓
↑
↑
1s 2s 2p
This can be converted into a Bohr model diagram.
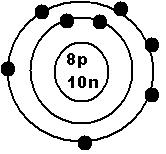
Each number (such as the 1 in 1s or the 2 in 2p) related to the ring number (or energy level) that those electrons are on. Start with protons and neutrons in the nucleus, then draw the electrons in rings around the outside. Note that all the electrons for a specific energy level go on the same ring, so the 2s and 2p electrons are all on the second ring.
EXAMPLE: This one's a bit more challenging.
110PdProtons = 46
Electrons = 46
Neutrons = 110 - 46 = 64
↑↓
↑↓
↑↓
↑↓
↑↓
↑↓
↑↓
↑↓
↑↓
↑↓
1s
2s
2p
3s
3p
4s
↑↓
↑↓
↑↓
↑↓
↑↓
↑↓
↑↓
↑↓
↑↓
↑↓
↑↓
↑↓
↑
↑
3d
4p
5s
4d
Bohr Model:
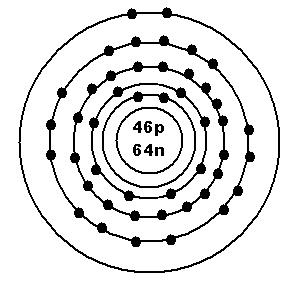
Notice that there are 5 rings with electrons, and the highest energy sublevel is 5s. Also, if you look at the periodic table, you will see that Pd is in the 5th period. So, the period number is equal to the number of shells that should appear in the ground state Bohr model. Note also that the d orbitals have 2 unpaired electrons. This is because they filled up with one electron in each orbital before they doubled up. Pd only has 2 valence electrons.