Oxidation-Reduction (REDOX) Reactions
- Oxidation-Reduction reactions are also known as REDOX reactions
- These involve the transfer of electrons (e-)
- Reduction is the gain of electrons
- The charge gets reduced (more negative) as electrons are added
- Oxidation is the loss of electrons
- Reduction and oxidation can each be represented by half-reactions
- Conservation of Charge applies
- The number of electrons lost must equal the number of electrons gained
Oxidation Numbers
- Basic oxidation numbers come from the group numbers.
- It's based on gaining or losing valence electrons to earn a full outer shell.
- Ex: Magnesium (group 2) has 2 valence electrons. It gives them away in an ionic bond to get a 2+ charge. This happens with all group 2 elements.
- Transition metals (groups 3-12) have varied oxidation states.
- All oxidation states are shown in the top right corners of your periodic table.
- Elements on their own in a reaction have an oxidation state of zero (0), even if they are diatomic.
General Oxidation Numbers
There is a general pattern of oxidation numbers for about half of the periodic table, as follows:
| Group | 1 | 2 | 3-12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Oxidation # | 1+ | 2+ | Varies | 3+ | 4+ | 3- | 2- | 21 | 0 |
Redox Reaction
Example reaction: H2 + F2 → 2HF
- Start by assigning charges (oxid #'s) to each element on both sides.
- Reactants H2 and F2 are each on their own, so they have oxidation numbers of 0.
- In HF, hydrogen has a 1+ charge and fluorine has a 1- charge (based on oxid #s).
- We use their oxidation numbers because they are bonded together.
- We can write a half-reaction for hydrogen that shows how its charge changes from 0 (in H2) to 1+ (in HF).
- To do this, we need to add electrons to one side to balance the charges.
- A second half-reaction can be created for fluorine.
Let's breakdown the reaction: hydrogen
First, show all the charges above each element in the reaction.
0 0 1+ 1-
H2 + F2 → 2HF
The charges on each atom are shown above. Let's use these to make the half-reactions.
H2 → 2H+
The problem is, this isn't balanced by charge. The charge on the left is zero and the total charge on the right is 2+ (because there are two H+).
To balance this, we need to add 2 electrons to the right side.
H2 → 2H+ + 2e-
This shows electrons being lost, therefore this is oxidation.
Let's breakdown the reaction: fluorine
First, show all the charges above each element in the reaction.
0 0 1+ 1-
H2 + F2 → 2HF
The charges on each atom are shown above. Let's use these to make the half-reactions.
F2 → 2F-
The problem is, this isn't balanced by charge. The charge on the left is zero and the total charge on the right is 2- (because there are two F-).
To balance this, we need to add 2 electrons to the left side.
F2 + 2e- → 2F-
This shows electrons being added, therefore this is reduction.
Final comments
H2 + F2 → 2HF
So, the two half-reactions for this reaction are:
oxidation: H2 → 2H+ + 2e-
reduction: F2 + 2e- → 2F-
If you were to add these two reactions together, the electrons would cancel out and you would end up with the original reaction.
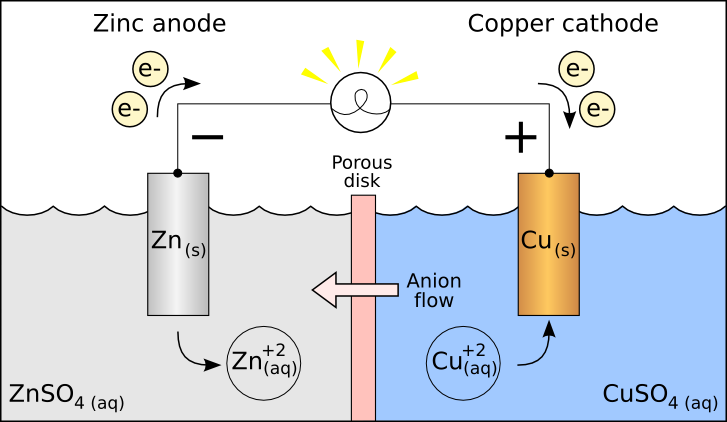
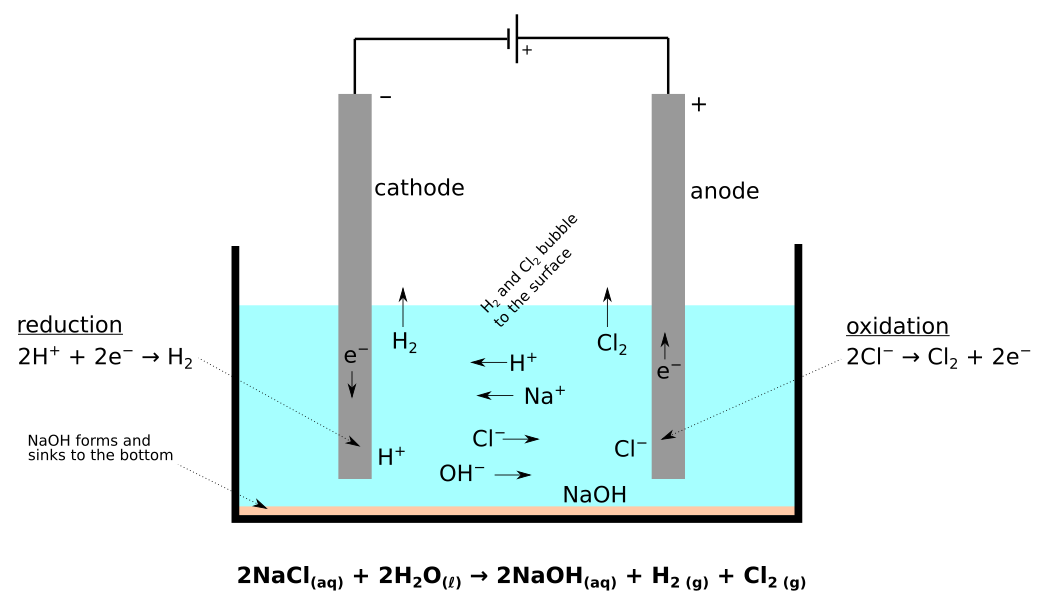
Electrochemical cells
-
Redox reactions can take place in an electrochemical cell
- This is just a place where electrons move between chemicals
- Conversation between chemical and electrical energy
- Anode versus Cathode
- Oxidation occurs at the Anode (vowels)
- Reduction occurs at the Cathode (consonants)
- There are 2 types of electrochemical cells
- Voltaic
- spontaneous, happens on its own
- Electrolytic
- requires the addition of electricity to produce chemical change
- this process is called electrolysis
- Voltaic
Voltaic Cell
|

|
Electrolytic Cell
|
|