Solubility Curves
Solubility
- Solubility is the ability of one substance (solute) to dissolve in another substance (solvent) in order to make a solution
- "Like dissolves like"
- Polar solvents can dissolve polar solutes
- salt in water
- Nonpolar solvents can dissolve nonpolar solutes
- oil dissolves in benzene
- Polar and nonpolar solutes and solvents will not dissolve
- Polar solvents can dissolve polar solutes
|
A solubility curve shows how much of a particular solute that will dissolve in a solvent at various temperatures. Generally, a solubility graph includes information from several solutes, such as shown in the image to the right. Reading the curve is easy. It reads like any other graph. You can look up the amount of solute based on the temperature, or you can look up the temperature based on the amount of solute. The most challenging part of reading the solubility graph is that several solutes appear. Look at that graph to the right. How many grams of KNO3 can dissolve in 100 grams of H2O at 60 °C? Look carefully and you should see that it reads 90 grams. |
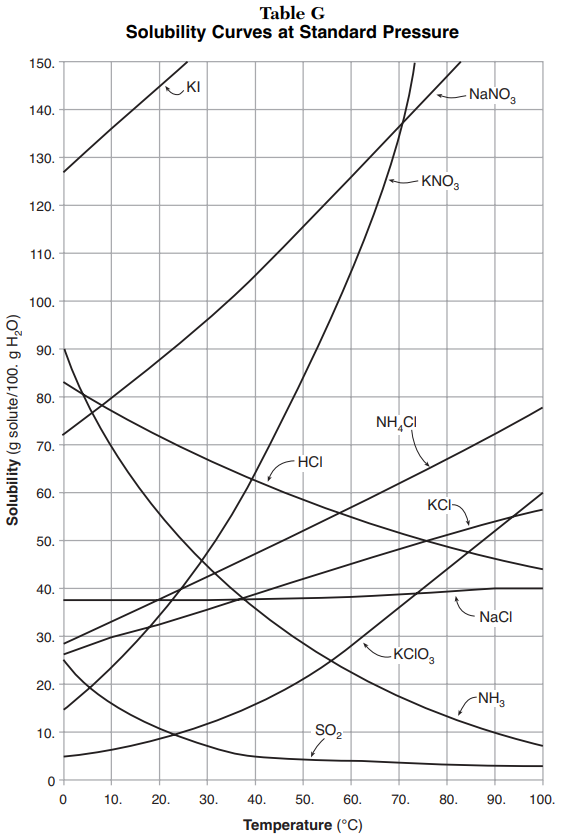 |
| Amount of Solvent | This solubility curve is based on 100 g of H2O as the solvent. If you have a different amount of solvent, you need to adjust the amount of solute. If you wanted to know how much KNO3 can dissolve in 200g H2O at 60°C, then you look up the information for 100 g of H2O then double it (because you have twice as much water/solvent). So: 90g x 2 = 180 grams |
| Unsaturated | A solution is unsaturated if is there is not enough solute to fill the solvent. Any amount of solute that falls below the solubility line, then the solution is unsaturated. Therefore, at 60°C, dissolving 50 grams of KNO3 in 100 grams of H2O would be an unsaturated solution. |
| Saturated | A solution is saturated if the solvent cannot dissolve any more solute. If the amount of solute falls on or above the solubility line, then the solution is saturated. Therefore, at 60°C, dissolving 120 grams of KNO3 in 100 grams of H2O would be a saturated solution. |
| Supersaturated |
You cannot tell if a solution is supersaturated from its solubility curve. |
| Precipitate | Amount of excess solute in the mixture. Simply look at the amount of solute being added in and subtract out the soluble amount. If your answer is negative, then there is no precipitate because the solution is unsaturated. |
| Additional solute | You can determine the addition amount of solute that can be added in a process similar to finding the precipitate. For an unsaturated solution, subtract the amount of solute used from the amount of solute that is possible to dissolve. |
Practice Problems
|

|
Solubility Calculator
This calculator can find answers to questions related to amount of solute, temperature,
saturation, and
precipitation of single solutes.
Temperature range: 10° - 100°. Solute range: 5g - 150g. Note: Water amounts
other than 100g may
currently cause erroneous answers for temperature.
Also, for water amounts other than 100g, the auto-grapher will show the adjusted solubility
amount (ie, what it
would be in 100 g water).
Starting Information
Input what you know. Leave the rest blank.
Solute:gTemperature:°C
Amount of H2O:g