Phase Diagrams
Heating Curve
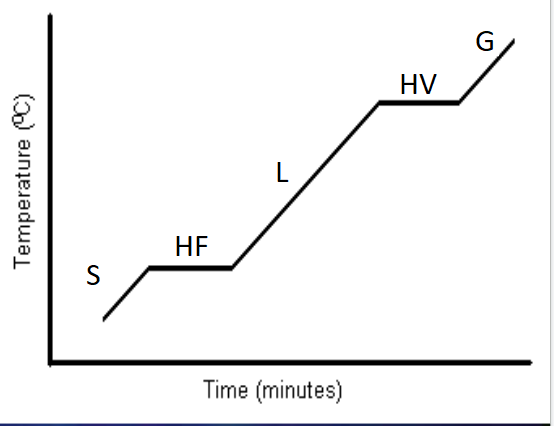
- S = Solid phase
- HF = Heat of Fusion
- energy between S and L; at equilibrium
- Heat of Fusion is the amount of energy required to change from solid to liquid. It is also the amount of energy lost when changing from liquid to solid.
- L = Liquid phase
- HV = Heat of Vaporization
- energy between G and L; at equilibrium
- Heat of Vaporization is the amount of energy required to change from liquid to gas. It is also the amount of energy lost when changing from gas to liquid.
- G = Gas phase
The lower down the curve, the lower the kinetic energy.
Going right across the flat lines, potential energy increases.
Heats can be calculated at various points along the curve.
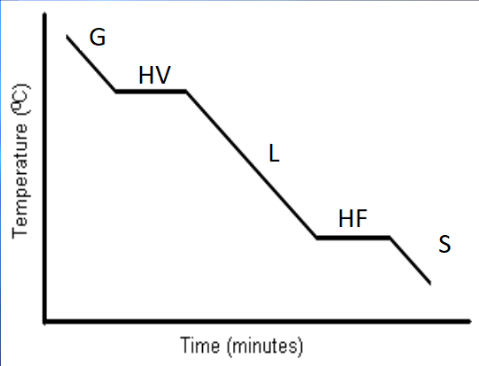
Cooling Curve
Same concept as the heating curve, just in reverse.
Heat Equations
- Heats can be calculated at different places on the heating curve
- Heat values are given on Reference Table B
- Formulas are given on Reference Table T
- Phase Changes:
- Fusion (S ⇄ L): q = mHf
- q = heat, m = mass, Hf = heat of fusion (334 kJ/mol)
- Vaporization (L ⇄ G): q = mHv
- q = heat, m = mass, Hv = heat of vaporization (2260 kJ/mol)
- Fusion (S ⇄ L): q = mHf
- Temperature Changes
- Temperature change: q=mCΔT
- q = heat, m = mass, C = specific heat capacity
- ΔT = change in temp (final-initial)
- C changes based on the phase of matter (4.18 kJ/kg oC for liquid H2O)
- Solid (ice) = 2.095 kJ/kgoC
- Liquid (water) = 4.18 kJ/kgoC
- Gas (steam) = 2.0267 kJ/kgoC
- Temperature change: q=mCΔT